Фарлетузумаб эктерибулин
Farletuzumab ecteribulinМНН
Rec. INN (наименование, зарегистрированное ВОЗ)
CAS
2407465-18-1
Химическое название
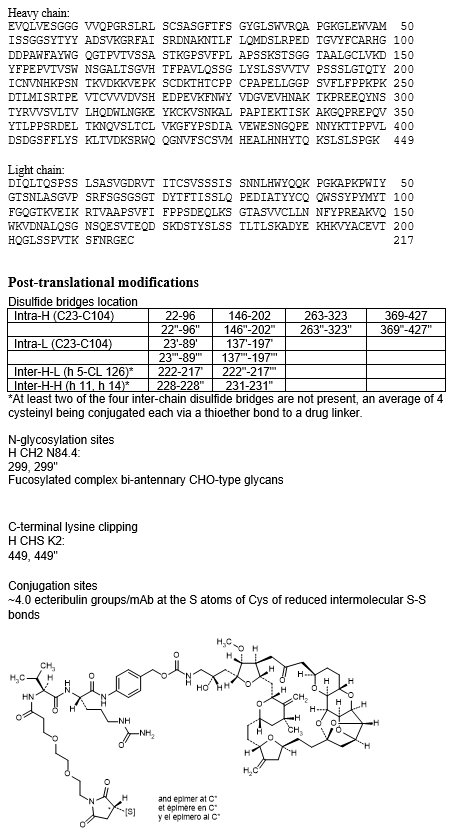
immunoglobulin G1-kappa, anti-[Homo sapiens FOLR1 (folate receptor 1, folate receptor alpha, FR-alpha, adult folate-binding protein, FBP, ovarian tumor-associated antigen MOv18)], humanized monoclonal antibody, conjugated to eribulin via a cleavable linker;
gamma1 heavy chain humanized (1-449) [VH (Homo sapiens IGHV3-30*03 (83.5%) -(IGHD) -IGHJ6*01 (90.9%) T123>P (114), CDR-IMGT [8.8.12] (26-33.51-58.97-108)) (1-119) -Homo sapiens IGHG1*01 (100%), G1m17,1 (CH1 K120 (216) (120-217), hinge 1-15 (218-232), CH2 (233-342), CH3 D12 (358), L14 (360) (343-447), CHS (448-449)) (120-449)], (222-217')-disulfide with kappa light chain humanized (1'-217') [V-KAPPA (Homo sapiens IGKV1-13*02 (81.2%) -IGKJ2*01 (91.7%) L124>V (107), CDR-IMGT [7.3.11] (27-33.51-53.90-100)) (1'-110') -Homo sapiens IGKC*01 (100%), Km3 A45.1 (156), V101 (194) (111'-217')]; dimer (228-228'':231-231'')-bisdisulfide, produced in a Chinese hamster ovary (CHO)-K1SV cell line, glycoform alfa; substituted at an average of four S atoms of cysteine residues (reduced inter-chain disulfide bonds) with (3RS)-1-[(6S,9S)-1-amino-6-[(4-{[({(2S)-2-hydroxy-3-[(12S,13S,14R,15R,32R,34R,36S,62S,65S,92S,93aR,94aR,95S,95aS,97R,99aS,910aR,910bS)-14-methoxy-34-methyl-33,63-bis(methylidene)-11-oxo-9-decahydro-93H-9(2,7)-(2,5-epoxyfuro[2',3':4,5]furo[3,2-b]pyrano[2,3-e]pyrana)-3(2,6)-oxana-1(2,3),6(2,5)-bis(oxolana)cyclododecaphan-15-yl]propyl}carbamoyl)oxy]methyl}phenyl)carbamoyl]-1,8,11-trioxo-9-(propan-2-yl)-14,17-dioxa-2,7,10-triazanonadecan-19-yl]-2,5-dioxopyrrolidin-3-yl (ecteribulin) groups
gamma1 heavy chain humanized (1-449) [VH (Homo sapiens IGHV3-30*03 (83.5%) -(IGHD) -IGHJ6*01 (90.9%) T123>P (114), CDR-IMGT [8.8.12] (26-33.51-58.97-108)) (1-119) -Homo sapiens IGHG1*01 (100%), G1m17,1 (CH1 K120 (216) (120-217), hinge 1-15 (218-232), CH2 (233-342), CH3 D12 (358), L14 (360) (343-447), CHS (448-449)) (120-449)], (222-217')-disulfide with kappa light chain humanized (1'-217') [V-KAPPA (Homo sapiens IGKV1-13*02 (81.2%) -IGKJ2*01 (91.7%) L124>V (107), CDR-IMGT [7.3.11] (27-33.51-53.90-100)) (1'-110') -Homo sapiens IGKC*01 (100%), Km3 A45.1 (156), V101 (194) (111'-217')]; dimer (228-228'':231-231'')-bisdisulfide, produced in a Chinese hamster ovary (CHO)-K1SV cell line, glycoform alfa; substituted at an average of four S atoms of cysteine residues (reduced inter-chain disulfide bonds) with (3RS)-1-[(6S,9S)-1-amino-6-[(4-{[({(2S)-2-hydroxy-3-[(12S,13S,14R,15R,32R,34R,36S,62S,65S,92S,93aR,94aR,95S,95aS,97R,99aS,910aR,910bS)-14-methoxy-34-methyl-33,63-bis(methylidene)-11-oxo-9-decahydro-93H-9(2,7)-(2,5-epoxyfuro[2',3':4,5]furo[3,2-b]pyrano[2,3-e]pyrana)-3(2,6)-oxana-1(2,3),6(2,5)-bis(oxolana)cyclododecaphan-15-yl]propyl}carbamoyl)oxy]methyl}phenyl)carbamoyl]-1,8,11-trioxo-9-(propan-2-yl)-14,17-dioxa-2,7,10-triazanonadecan-19-yl]-2,5-dioxopyrrolidin-3-yl (ecteribulin) groups
Структура
Иностранные названия
- Farletuzumabum ecteribulinum (латинское)
- Farletuzumab ecteribulin (английское)
- Farletuzumab ecteribulin (немецкое)
- Farlétuzumab ectéribuline (французское)
- Farletuzumab ecteribulina (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Фарлетузумаб эктерибулин:
