Лувелтамаб тазевибулин
Luveltamab tazevibulinМНН
Rec. INN (Recommended International Nonproprietary Name) — рекомендованное международное непатентованное наименование
CAS
2493327-62-9
Химическое название
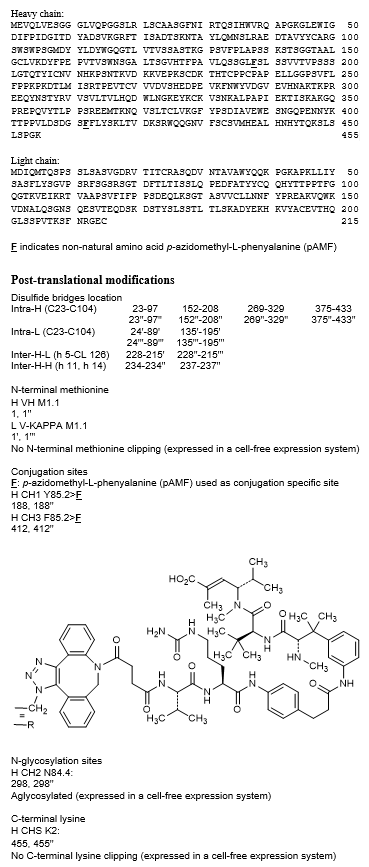
immunoglobulin G1-kappa, anti-[Homo sapiens FOLR1 (folate receptor 1, folate receptor alpha, FR-alpha, adult folate-binding protein, FBP, ovarian tumor-associated antigen MOv18)], humanized monoclonal antibody, conjugated on four modified phenylalanine residues via a cleavable valyl-citrullyl linker with a hemiasterlin analogue;
gamma1 heavy chain humanized (1-455) [VH (Homo sapiens IGHV3-66*01 (79.6%) -(IGHD) -IGHJ4*01 (93.3%), CDR-IMGT [8.8.17] (27-34.52-59.98-114)) (1-125) -Homo sapiens IGHG1*03v, G1m3>G1m17, nG1m1 (CH1 Y85.2>F (pAMF) (188), R120>K (222) (126-223), hinge 1-15 (224-238), CH2 (239-348), CH3 E12 (364), M14 (366), F85.2> F (pAMF) (412) (349-453), CHS (454-455)) (126-455)], (228-215')-disulfide with kappa light chain humanized (1'-215') [V-KAPPA (Homo sapiens IGKV1-39*01 (86.3%) -IGKJ1*01 (100%), CDR-IMGT [6.3.9] (28-33.51-53.90-98)) (1'-108') -Homo sapiens IGKC*01 (100%), Km3 A45.1 (154), V101 (192) (109'-215')]; dimer (234-234'':237-237'')-bisdisulfide, produced by a cell-free protein synthesis system based on Escherichia coli lysate, non-glycosylated, conjugated at C-4 of the four l-phenylalanyl residues 188, 412, 188'' and 412'' with [8-(4-{[(2S)-1-{[(2S)-5-(carbamoylamino)-1-(4-{[({3-[(3S)-4-{[(2S)-1-{[(3S,4E)-5-carboxy-2-methylhex-4-en-3-yl](methyl)amino}-3,3-dimethyl-1-oxobutan-2-yl]amino}-2-methyl-3-(methylamino)-4-oxobutan-2-yl]phenyl}carbamoyl)oxy]methyl}anilino)-1-oxopentan-2-yl]amino}-3-methyl-1-oxobutan-2-yl]amino}-4-oxobutanoyl)-8,9-dihydro-1H(or 3H)-dibenzo[b,f][1,2,3]triazolo[4,5-d]azocin-1(or 3)-yl]methyl (tazevibulin) groups
gamma1 heavy chain humanized (1-455) [VH (Homo sapiens IGHV3-66*01 (79.6%) -(IGHD) -IGHJ4*01 (93.3%), CDR-IMGT [8.8.17] (27-34.52-59.98-114)) (1-125) -Homo sapiens IGHG1*03v, G1m3>G1m17, nG1m1 (CH1 Y85.2>F (pAMF) (188), R120>K (222) (126-223), hinge 1-15 (224-238), CH2 (239-348), CH3 E12 (364), M14 (366), F85.2> F (pAMF) (412) (349-453), CHS (454-455)) (126-455)], (228-215')-disulfide with kappa light chain humanized (1'-215') [V-KAPPA (Homo sapiens IGKV1-39*01 (86.3%) -IGKJ1*01 (100%), CDR-IMGT [6.3.9] (28-33.51-53.90-98)) (1'-108') -Homo sapiens IGKC*01 (100%), Km3 A45.1 (154), V101 (192) (109'-215')]; dimer (234-234'':237-237'')-bisdisulfide, produced by a cell-free protein synthesis system based on Escherichia coli lysate, non-glycosylated, conjugated at C-4 of the four l-phenylalanyl residues 188, 412, 188'' and 412'' with [8-(4-{[(2S)-1-{[(2S)-5-(carbamoylamino)-1-(4-{[({3-[(3S)-4-{[(2S)-1-{[(3S,4E)-5-carboxy-2-methylhex-4-en-3-yl](methyl)amino}-3,3-dimethyl-1-oxobutan-2-yl]amino}-2-methyl-3-(methylamino)-4-oxobutan-2-yl]phenyl}carbamoyl)oxy]methyl}anilino)-1-oxopentan-2-yl]amino}-3-methyl-1-oxobutan-2-yl]amino}-4-oxobutanoyl)-8,9-dihydro-1H(or 3H)-dibenzo[b,f][1,2,3]triazolo[4,5-d]azocin-1(or 3)-yl]methyl (tazevibulin) groups
Структура
Иностранные названия
- Luveltamabum tazevibulinum (латинское)
- Luveltamab tazevibulin (английское)
- Luveltamab tazevibulin (немецкое)
- Luveltamab tazévibuline (французское)
- Luveltamab tazevibulina (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Лувелтамаб тазевибулин:
