Нулабеглоген аутогедтемцел
Nulabeglogene autogedtemcelМНН
Rec. INN (наименование, зарегистрированное ВОЗ)
Химическое название
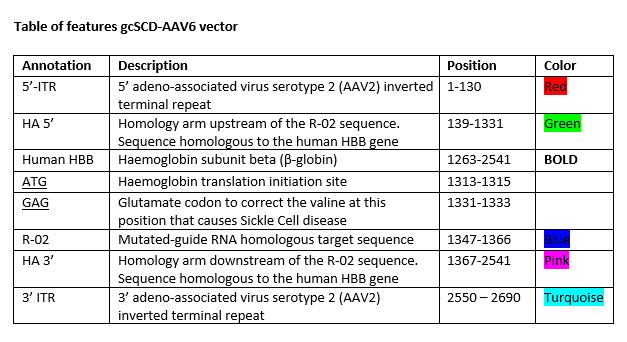
autologous CD34+ hematopoietic stem and progenitor cells (HSPCs) obtained by apheresis from sickle cell patients, genetically modified ex vivo by CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9)-mediated gene editing consisting of a single guide RNA (sgRNA) targeting the first exon of the human β-globin (HBB) gene, and using a homology-directed repair mechanism to correct the nucleic acid sequence encoding the glutamic acid to valine mutation at position 6 in the HBB protein via an adeno-associated virus serotype 6 (AAV6) vector in which the corrective sequence is located between two HBB homology arms. Following genetic modification, each cell can have one of six combinations of HBB allele (WT/WT, INDEL/WT, INDEL/INDEL, INDEL/HR, WT/HR, HR/HR, where WT refers to the wild type sickle cell disease allele, INDEL refers to an insertion/deletion event but no correction of the HBB gene, and HR refers to a corrected HBB allele). The final substance consists of cells with ≥ 20% alleles that are corrected of the E6V mutation, measured by the frequency of homologous recombination events. The cell suspension is enriched for CD34+ cells using magnetic bead separation. The substance consists of cells with the CD45+ and CD34+ phenotype, with ≥80% CD34+ purity. The functional characterization of the cells is based on the ability to form erythroid and myeloid colonies on semisolid methylcellulose-based medium
Структура



Иностранные названия
- Nulabeglogenum autogedtemcelum (латинское)
- Nulabeglogene autogedtemcel (английское)
- Nulabeglogen autogedtemcel (немецкое)
- Nulabéglogène autogedtemcel (французское)
- Nulabeglogén autogedtemcel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Нулабеглоген аутогедтемцел:
