Рибрекабтаген аутолейцел
Ribrecabtagene autoleucelМНН
Rec. INN (наименование, зарегистрированное ВОЗ)
Химическое название
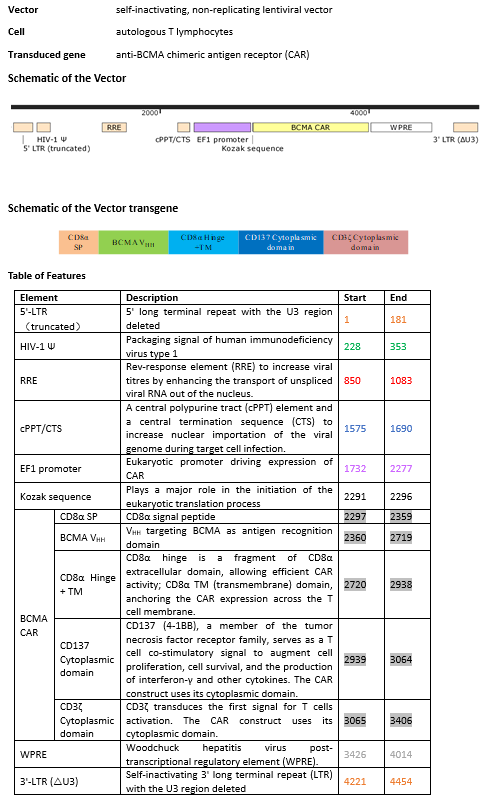
autologous T lymphocytes obtained from peripheral blood by leukapheresis, transduced with a self-inactivating, non- replicating lentiviral vector encoding a chimeric antigen receptor (CAR) targeting the B-cell maturation antigen (BCMA). The expressed transgene comprises a CD8α leader sequence, an anti-BCMA humanized camelid single domain antibody (VHH), a CD8α hinge and transmembrane domain, and a 4-1BB co-stimulatory domain and CD3ζ signalling domain, under control of the human elongation factor 1 (EF-1) promoter. The construct is flanked by 5' and 3' long terminal repeats (LTRs) and also contains a ψ packaging signal, a Rev response element (RRE), a central polypurine tract (cPPT) sequence and a Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE). The vector is pseudotyped with vesicular stomatitis virus (VSV) G glycoprotein.The leukapheresis material is enriched for CD3+ T lymphocytes by positive immunoselection. The lymphocytes are then activated by culture in bags coated with anti-CD3 antibody and recombinant human fibronectin fragment, followed by transduction with the lentiviral vector. The cells are further culture-expanded in growth media containing interleukin 2 (IL-2) and inactivated autologous plasma. The cell suspension consists of T lymphocytes (>95%), with greater than 10% of the T lymphocytes expressing the CAR-BCMA transgene. The transduced T lymphocytes demonstrate cytotoxicity against BCMA-expressing cells (>20%)
Структура
Иностранные названия
- Ribrecabtagenum autoleucelum (латинское)
- Ribrecabtagene autoleucel (английское)
- Ribrecabtagen autoleucel (немецкое)
- Ribrécabtagène autoleucel (французское)
- Ribrecabtagén autoleucel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Рибрекабтаген аутолейцел:
