Сатрикабтаген аутолейцел
Satricabtagene autoleucelМНН
Rec. INN (Recommended International Nonproprietary Name) — рекомендованное международное непатентованное наименование
Химическое название
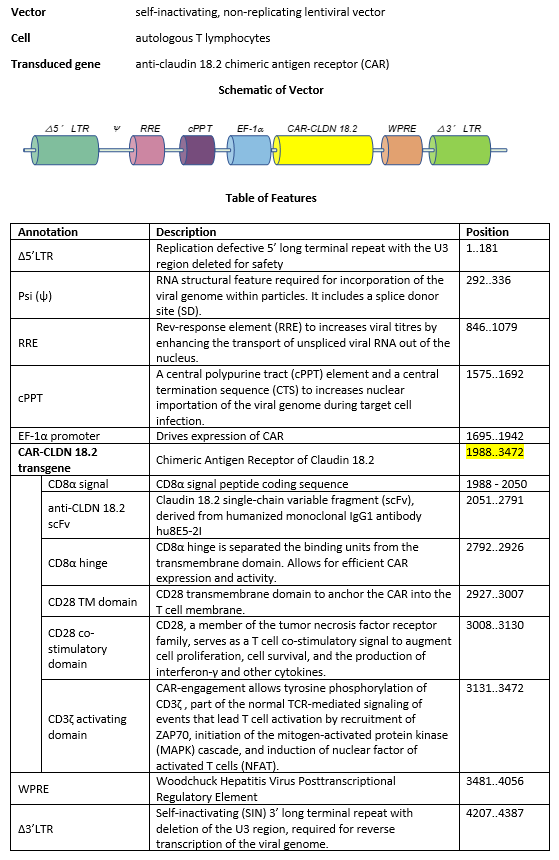
autologous T lymphocytes obtained from peripheral blood mononuclear cells by leukapheresis, transduced with a self- inactivating, non-replicating lentiviral vector, encoding a chimeric antigen receptor (CAR) targeting human claudin 18.2 (also known as claudin 18 isoform A2, CLDN18.2). The expressed transgene comprises anti-human CLDN18.2 single-chain variable fragment (scFv), derived from humanized monoclonal IgG1 antibody hu8E5-2I, human CD8α hinge, CD28 transmembrane and co-stimulatory domain and CD3ζ signalling domain and is under control of the elongation factor 1 alpha (EF1α) promoter. The construct is flanked by 5' and 3' long terminal repeats (LTRs) and also contains a ψ packaging signal, a Rev response element (RRE), a central polypurine tract (cPPT) sequence and a Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE).
The leukapheresis material is activated by CD3 and CD28 agonists and transduced with the lentiviral vector. The cells are then expanded in media supplemented with autologous serum and interleukin 2 (IL-2). The cell suspension consists of T lymphocytes (>70%), with greater than 10% of the T lymphocytes expressing the CAR transgene and secreting interferon gamma (IFN-γ) following co-culture with claudin 18.2-expressing cells.
The leukapheresis material is activated by CD3 and CD28 agonists and transduced with the lentiviral vector. The cells are then expanded in media supplemented with autologous serum and interleukin 2 (IL-2). The cell suspension consists of T lymphocytes (>70%), with greater than 10% of the T lymphocytes expressing the CAR transgene and secreting interferon gamma (IFN-γ) following co-culture with claudin 18.2-expressing cells.
Структура
Иностранные названия
- Satricabtagenum autoleucelum (латинское)
- Satricabtagene autoleucel (английское)
- Satricabtagen autoleucel (немецкое)
- Satricabtagène autoleucel (французское)
- Satricabtagén autoleucel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Сатрикабтаген аутолейцел:
