Такатресген аутолейцел
Tacatresgene autoleucelМНН
Rec. INN (наименование, зарегистрированное ВОЗ)
Химическое название
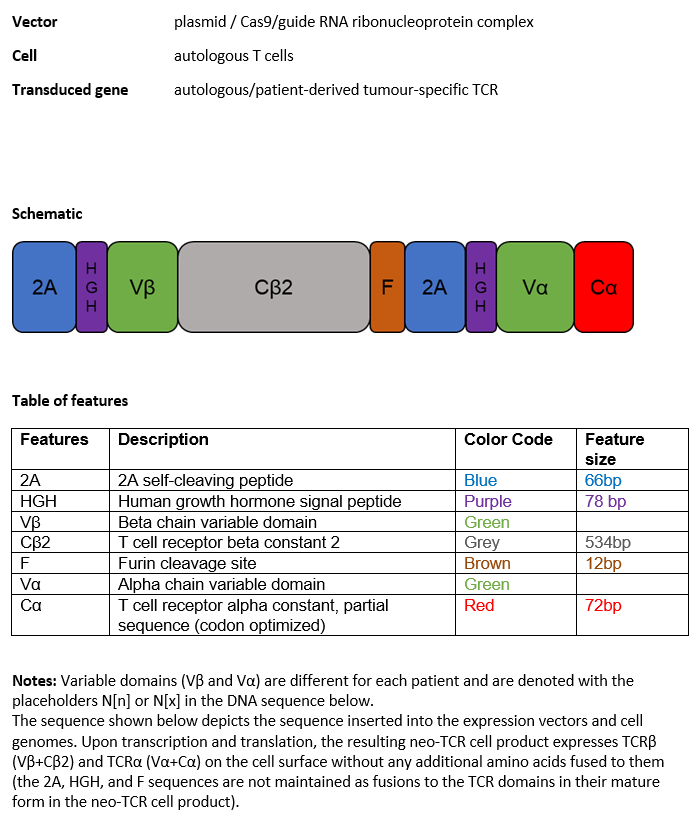
Autologous T cells obtained from the peripheral blood of patients, collected by leukapheresis, modified by CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9) mediated gene editing consisting of two guide RNAs (gRNAs) introduced transiently as ribonucleoprotein (RNP) complex, and a neoepitope T cell receptor (neoTCR) gene cassette encoded by plasmid DNA to replace the endogenous T cell receptor (TCR) with a single patient-derived tumour-specific neo-TCR per T cell, with a maximum of three different neo-TCRs in the final drug substance. The constant elements of the neo-TCR are identical for each patient and the neo-TCR gene expression is regulated by the native endogenous TCR promoter. The T cells are cultured in the presence of growth media containing IL-7 and IL-15 and a combination of membrane bound anti-CD3 and anti-CD28 for activation. The T cells are predominantly CD4 and CD8 T cells (generally >80%), including Tmsc (memory stem cells) and Tcm (central memory) phenotypes.
Структура

Иностранные названия
- Tacatresgenum autoleucelum (латинское)
- Tacatresgene autoleucel (английское)
- Tacatresgen autoleucel (немецкое)
- Tacatresgène autoleucel (французское)
- Tacatresgén autoleucel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Такатресген аутолейцел:
