Таликабтаген аутолейцел
Talicabtagene autoleucelМНН
Rec. INN (Recommended International Nonproprietary Name) — рекомендованное международное непатентованное наименование
Химическое название
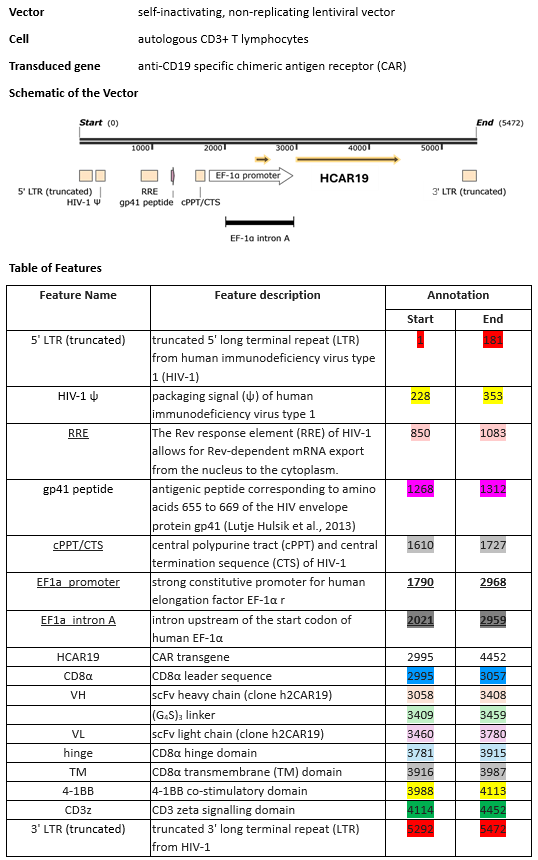
autologous peripheral blood mononuclear cells (PBMCs) obtained by leukapheresis enriched for CD3+ T lymphocytes, transduced with a self-inactivating non-replicating lentiviral vector encoding a chimeric antigen receptor (CAR) targeting CD19, comprising a CD8α leader sequence, a humanised anti-CD19 single chain variable fragment (scFv; derived from the FMC63 antibody), human CD8α hinge and transmembrane domain, human 4-1BB co-stimulatory domain and human CD3ζ signalling domain, under control of the elongation factor-1 alpha (EF-1α) promoter. The vector genome also contains an HIV-1 packaging signal (ψ), a Rev response element (RRE), a central polypurine tract (cPPT)/central termination sequence (CTS) and is flanked by 5' and 3' long terminal repeats (LTRs). The vector is pseudotyped with vesicular stomatitis virus (VSV) G glycoprotein., The leukapheresis material is enriched for CD3+ T lymphocytes by positive immunoselection, activated by CD3 and CD28 agonists and transduced with the lentiviral vector. The cells are then expanded in media supplemented with human AB serum and interleukin 2 (IL-2)., The T lymphocytes (≥95%) are positive for the transgene (≥15% CAR positive) and are cytotoxic (≥50%) when co-cultured with CD19 expressing tumour cell lines
Структура

Иностранные названия
- Talicabtagenum autoleucelum (латинское)
- Talicabtagene autoleucel (английское)
- Talicabtagen autoleucel (немецкое)
- Talicabtagène autoleucel (французское)
- Talicabtagén autoleucel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Таликабтаген аутолейцел:
