Тинокабтаген аутолейцел
Tinocabtagene autoleucelМНН
Rec. INN (наименование, зарегистрированное ВОЗ)
Химическое название
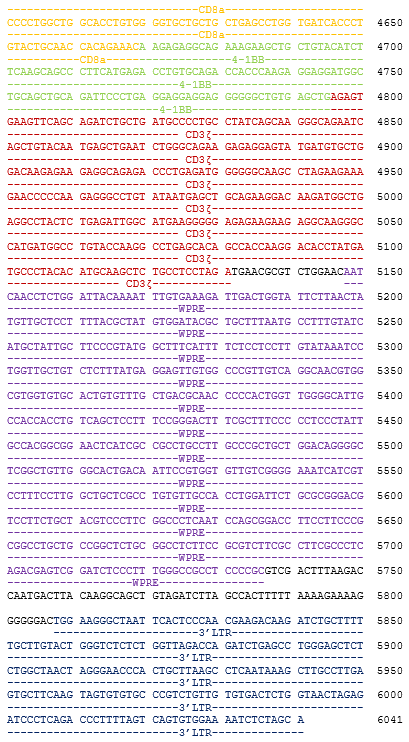
autologous T lymphocytes obtained from peripheral blood lymphocytes by leukapheresis, transduced with a self-inactivating, non-replicating lentiviral vector, encoding a bispecific chimeric antigen receptor targeting CD19 and CD22 (Siglec-2). The expressed transgene comprises a CD8α leader sequence, an anti- CD19 and anti-CD22 fully human single chain fragment variable (scFv), a CD8α hinge and transmembrane region, and a 4-1BB and CD3ζ signalling domain and is under control of the elongation factor 1 alpha (EF1α) promoter. The construct is flanked by 5' and 3' long terminal repeats (LTRs) and also contains a ψ packaging signal, a Rev response element (RRE), a central polypurine tract (cPPT) sequence and a mutated Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE). The vector is pseudotyped with vesicular stomatitis virus (VSV) G envelope protein. The leukapheresis material is enriched for CD4/CD8 T lymphocytes by positive immunoselection, activated by CD3 and CD28 agonists and transduced with the vector. The cells are then expanded in optimized serum-free cell culture media with serum replacement and interleukin 2 (IL-2). The T lymphocytes (≥90%; with <5% CD19+/CD22+ B cell impurity) are positive for the transgene (≥10% CAR positive) and secrete interferon gamma (IFN-γ)
Структура
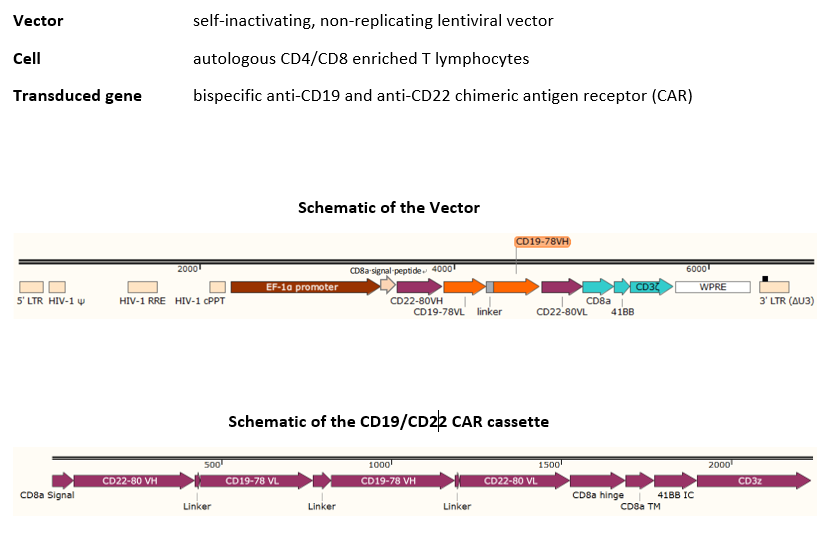
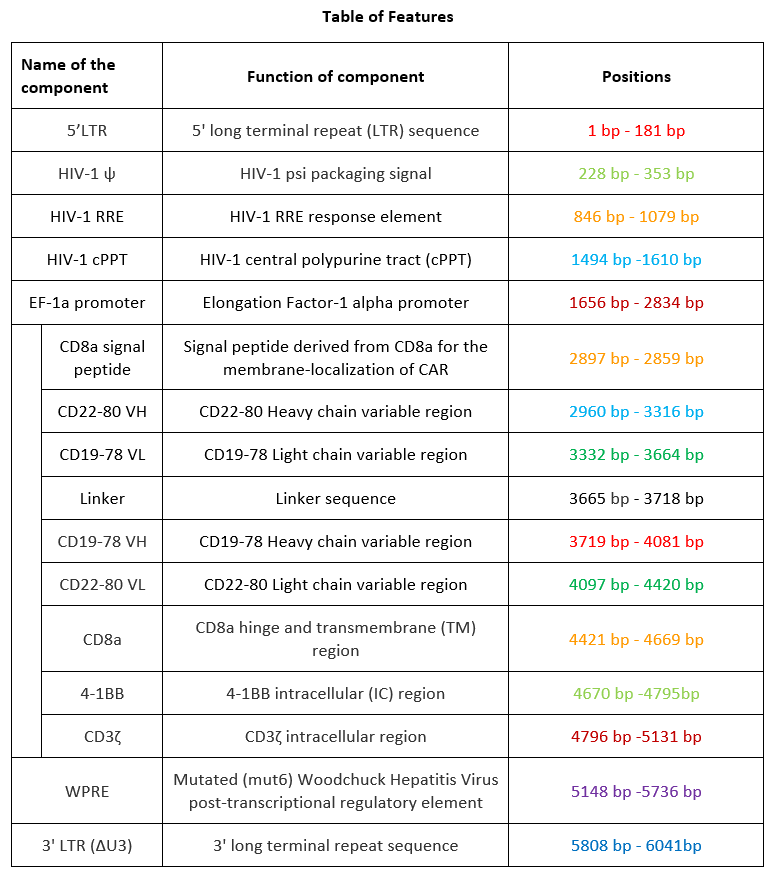
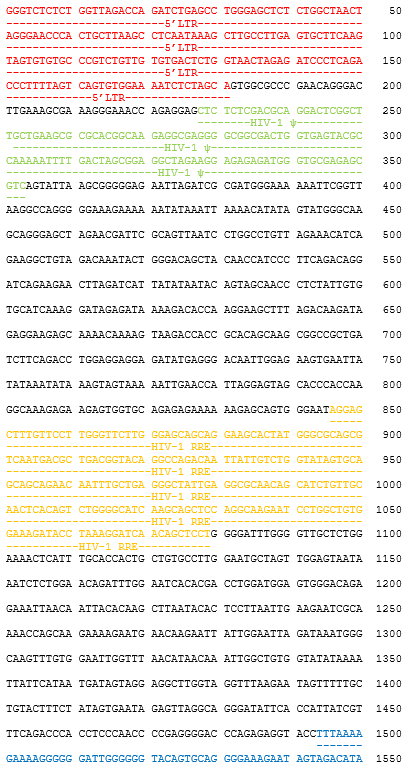

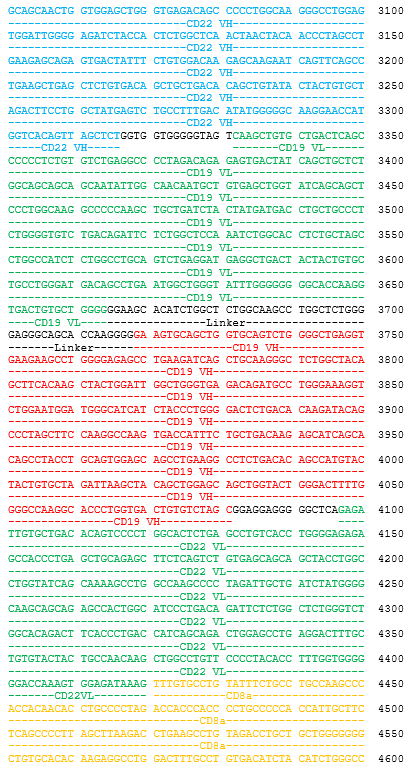
Иностранные названия
- Tinocabtagenum autoleucelum (латинское)
- Tinocabtagene autoleucel (английское)
- Tinocabtagen autoleucel (немецкое)
- Tinocabtagène autoleucel (французское)
- Tinocabtagén autoleucel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Тинокабтаген аутолейцел:
