Занидатамаб зоводотин
Zanidatamab zovodotinМНН
Rec. INN (наименование, зарегистрированное ВОЗ)
CAS
2329698-82-8
Химическое название
immunoglobulin half-IG G1-kappa/scFv-h-CH2-CH3, anti-[Homo sapiens ERBB2 (epidermal growth factor receptor 2, receptor tyrosine protein kinase erbB-2, EGFR2, HER2, HER-2, p185c-erbB2, NEU, CD340)], humanized monoclonal antibody, biparatopic (targeting two different non-overlapping epitopes on ERBB2, on extracellular domains 2 (ECD2) and 4 (ECD4)), conjugated to a derivative of auristatin;
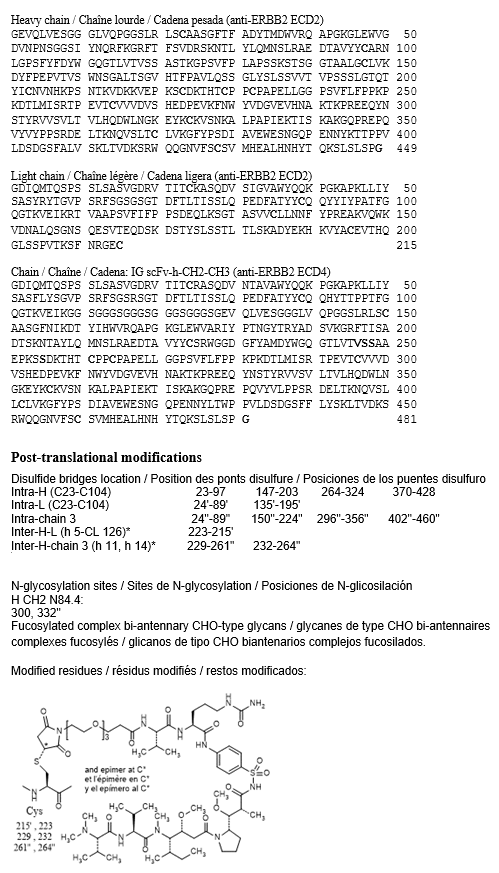
gamma1 heavy chain, anti-ERBB2 extracellular domain 2 (ECD2), humanized (1-449) [VH humanized (Homo sapiens IGHV3-66*01 (78.8%) -(IGHD) - IGHJ4*01 (100%)) CDR-IMGT[8.8.12] (27-34.52-59.98-109) (1-120) -Homo sapiens IGHG1*01 G1m17,1 (CH1 K120 (217) (121-218), hinge 1-15 (219- 233), CH2 (234-343), CH3 T6>V (353), L7>Y (354), D12 (359), L14 (361), F85.1>A (408), Y86>V (410) (344-448), CHS K2>del (449)) (121-449)], (223- 215')-disulfide with kappa light chain, anti ERBB2 ECD2, humanized (1’-215’) [V-KAPPA humanized (Homo sapiens IGKV1-16*01 (84.2%) -IGKJ1*01 (100%)) CDR-IMGT [6.3.9] (28-33.51-53.90-98) (1’-108’) -Homo sapiens IGKC*01 (100%) Km3 A45.1 (154), V101 (192) (109'-215')]; IG scFv-h-CH2-CH3 single chain, anti-ERBB2 extracellular domain 4 (ECD4), humanized (1”-481”) [scFv V-kappa-VH anti-ERBB2 ECD4 (1’-248') [V-KAPPA humanized (Homo sapiens IGKV1-39*01 (86.3%) -IGKJ1*01 (100%)) CDR- IMGT [6.3.9] (28-33.51-53.90-98) (1’-108’) -20-mer pentakis(diglycyl-seryl- glycyl) linker (109''-128'') -VH humanized (Homo sapiens IGHV3-66*01 (81.6%) -(IGHD) -IGHJ4*01 (100%)) CDR-IMGT [8.8.13] (154-161.179-186.225-237) (129''-248'')] -dialanyl linker (249''-250'') -Homo sapiens IGHG1*01 h-CH2-CH3, G1m1 (251''-481'') [hinge 1-15, C5>S (255) (251-265), CH2 (266-375), CH3 T6>V (385), D12 (391), L14 (393), T22>L (401), K79>L (427), T81>W (429) (376-480), CHS K2>del (481)]]; dimer (229-261'':232-264'')-bisdisulfide, produced in Chinese hamster ovary (CHO) cells, glycoform alfa, conjugated, on an average of 2 to 3 cysteinyl, to a sulfonamide containing auristatin derivative, via a cleavable 1-maleimido-3,6,9-trioxadodecan-12-oyl-valyl-citrullyl linker
gamma1 heavy chain, anti-ERBB2 extracellular domain 2 (ECD2), humanized (1-449) [VH humanized (Homo sapiens IGHV3-66*01 (78.8%) -(IGHD) - IGHJ4*01 (100%)) CDR-IMGT[8.8.12] (27-34.52-59.98-109) (1-120) -Homo sapiens IGHG1*01 G1m17,1 (CH1 K120 (217) (121-218), hinge 1-15 (219- 233), CH2 (234-343), CH3 T6>V (353), L7>Y (354), D12 (359), L14 (361), F85.1>A (408), Y86>V (410) (344-448), CHS K2>del (449)) (121-449)], (223- 215')-disulfide with kappa light chain, anti ERBB2 ECD2, humanized (1’-215’) [V-KAPPA humanized (Homo sapiens IGKV1-16*01 (84.2%) -IGKJ1*01 (100%)) CDR-IMGT [6.3.9] (28-33.51-53.90-98) (1’-108’) -Homo sapiens IGKC*01 (100%) Km3 A45.1 (154), V101 (192) (109'-215')]; IG scFv-h-CH2-CH3 single chain, anti-ERBB2 extracellular domain 4 (ECD4), humanized (1”-481”) [scFv V-kappa-VH anti-ERBB2 ECD4 (1’-248') [V-KAPPA humanized (Homo sapiens IGKV1-39*01 (86.3%) -IGKJ1*01 (100%)) CDR- IMGT [6.3.9] (28-33.51-53.90-98) (1’-108’) -20-mer pentakis(diglycyl-seryl- glycyl) linker (109''-128'') -VH humanized (Homo sapiens IGHV3-66*01 (81.6%) -(IGHD) -IGHJ4*01 (100%)) CDR-IMGT [8.8.13] (154-161.179-186.225-237) (129''-248'')] -dialanyl linker (249''-250'') -Homo sapiens IGHG1*01 h-CH2-CH3, G1m1 (251''-481'') [hinge 1-15, C5>S (255) (251-265), CH2 (266-375), CH3 T6>V (385), D12 (391), L14 (393), T22>L (401), K79>L (427), T81>W (429) (376-480), CHS K2>del (481)]]; dimer (229-261'':232-264'')-bisdisulfide, produced in Chinese hamster ovary (CHO) cells, glycoform alfa, conjugated, on an average of 2 to 3 cysteinyl, to a sulfonamide containing auristatin derivative, via a cleavable 1-maleimido-3,6,9-trioxadodecan-12-oyl-valyl-citrullyl linker
Структура
Иностранные названия
- Zanidatamabum zovodotinum (латинское)
- Zanidatamab zovodotin (английское)
- Zanidatamab zovodotin (немецкое)
- Zanidatamab zovodotine (французское)
- Zanidatamab zovodotina (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Занидатамаб зоводотин:
