Брексукабтаген аутолейсел
Brexucabtagene autoleucelМНН
Rec. INN (наименование, зарегистрированное ВОЗ)
Drugbank
DB15699
АТХ
Код МКБ 10
Категория при беременности по FDA
N (не классифицировано FDA)
Химическое название
autologous T cells obtained from the peripheral blood of patients with relapsed/refractory mantle cell lymphoma, collected by leukapheresis, transduced with a murine stem cell virus-based retroviral vector encoding a chimeric antigen receptor (CAR) under the control of the 5' long terminal repeat (LTR) murine stem cell virus promoter, comprising a single-chain variable fragment derived from the murine antibody FMC63 targeting CD19 (B-lymphocyte antigen CD19), a CD28 spacer, a CD28 transmembrane and intracellular costimulatory domains, and a CD3ζ intracellular activation domain. The T cells are selected with anti-CD4 and anti-CD8 coated particles, activated with anti-CD3, and anti-CD28 antibodies and cultured in media containing IL-2. The CD4/CD8 enriched T cells are predominantly CD3+ (≥98%), anti-CD19.CAR (≥24%), and contain on average 0.1% natural killer (NK) cells and 0.5% other cellular impurities. The T cells also release interferon gamma (IFN-γ) by co-culture in vitro.
Структура
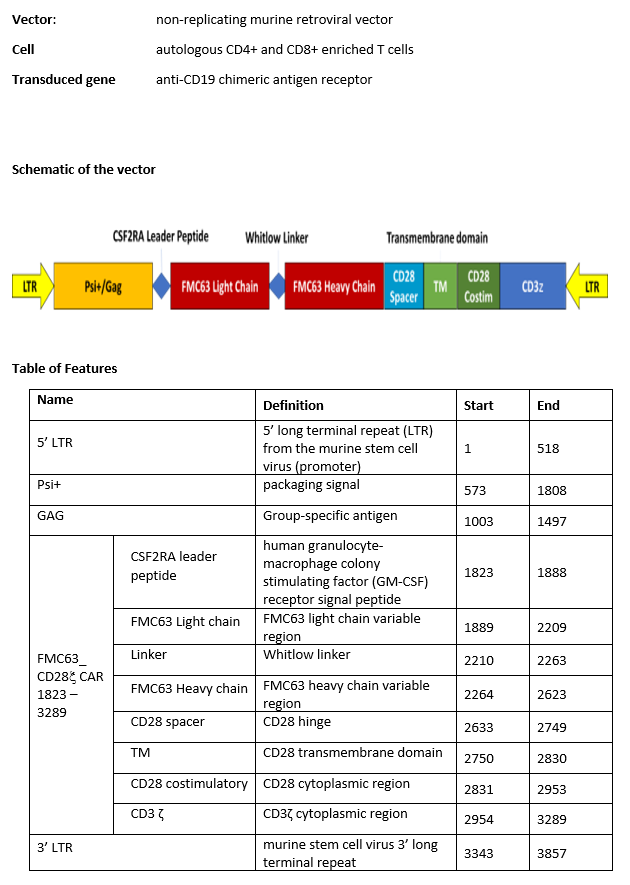
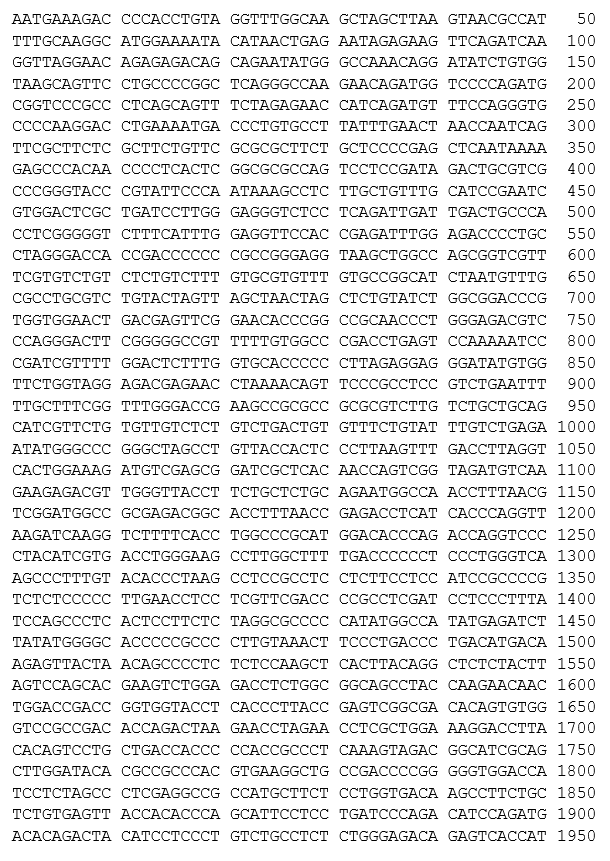
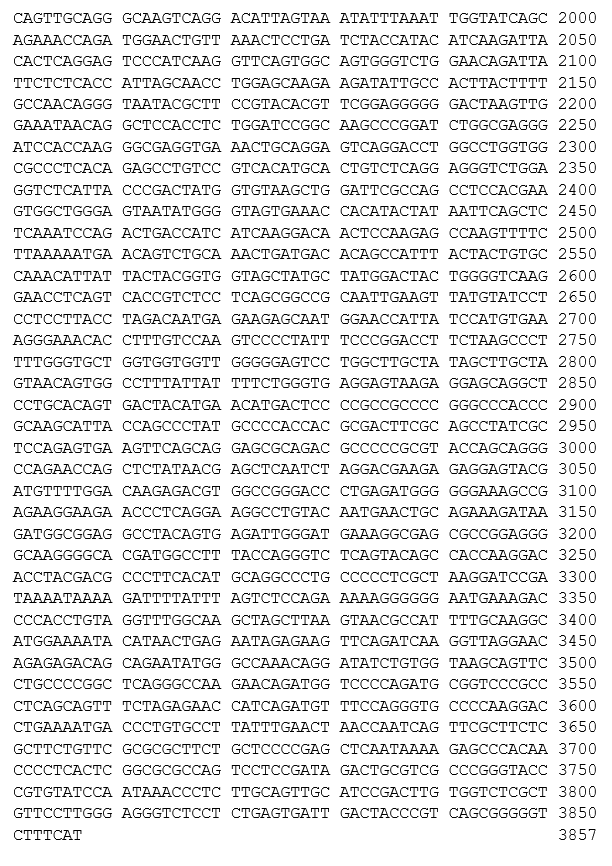
Иностранные названия
- Brexucabtagenum autoleucelum (латинское)
- Brexucabtagene autoleucel (английское)
- Bréxucabtagène autoleucel (французское)
- Brexucabtagén autoleucel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Брексукабтаген аутолейсел:
