Обекабтаген аутолейцел
Obecabtagene autoleucelМНН
Rec. INN (Recommended International Nonproprietary Name) — рекомендованное международное непатентованное наименование
PubChem
DB17362
АТХ
Категория при беременности по FDA
N (не классифицировано FDA)
Химическое название
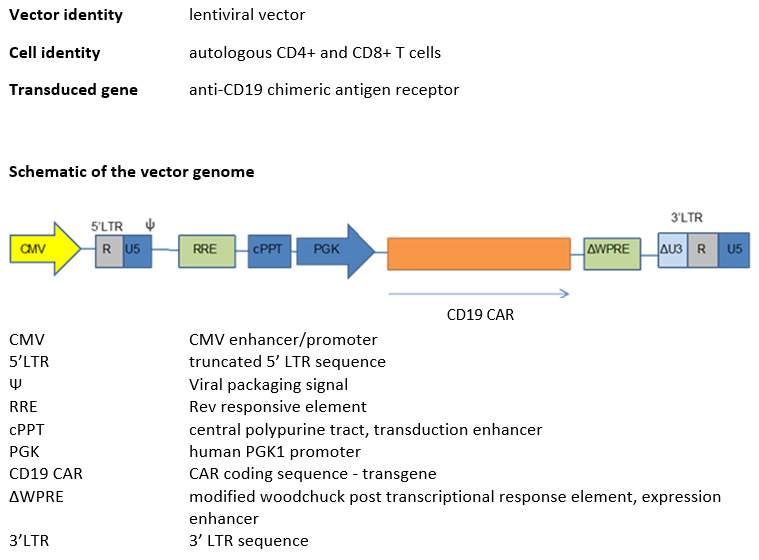
Autologous CD4+ and CD8+ T cells transduced ex vivo with a self-inactivating lentiviral vector encoding an anti-CD19 chimeric antigen receptor.
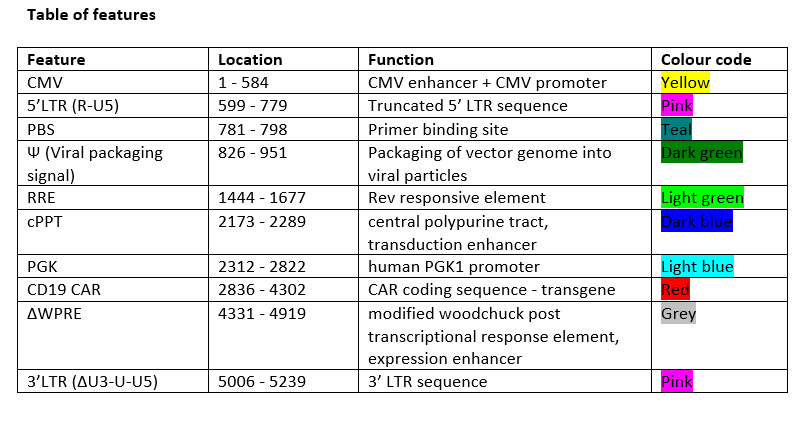
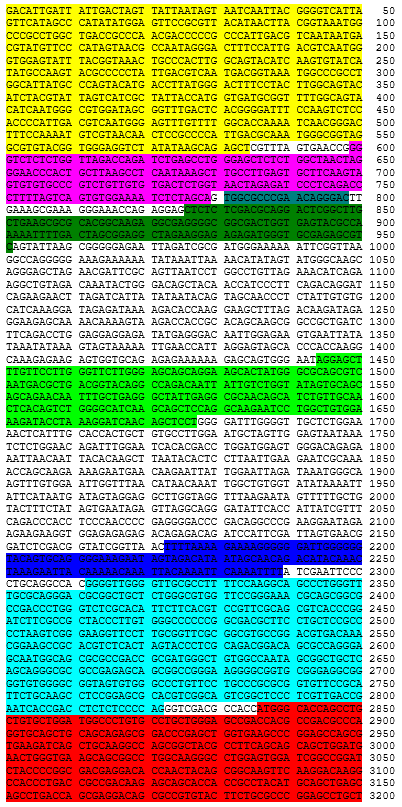
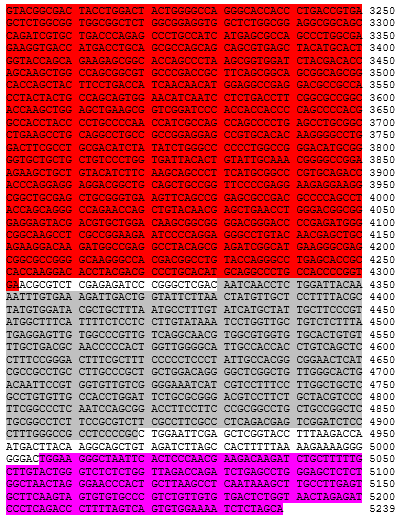
Autologous CD4+ and CD8+ T cells, enriched from peripheral blood mononuclear cells (PBMCs) obtained by apheresis transduced ex vivo with a non-replicating, self-inactivating (SIN) lentiviral vector, encoding a chimeric antigen receptor (CAR) consisting of an antiCD19 specific scFv, a CD8α stalk, transmembrane domain and anchor, a 41BB endodomain and a CD3 zeta endodomain, under the control of a human PGK1 promoter and a modified Woodchuck post transcriptional response element. The vector genome also contains a 5' terminal CMV enhancer/promoter, a Ψ packaging signal, a Rev response element (RRE) and a central polypurine tract (cPPT).
Autologous CD4+ and CD8+ T cells, enriched from peripheral blood mononuclear cells (PBMCs) obtained by apheresis transduced ex vivo with a non-replicating, self-inactivating (SIN) lentiviral vector, encoding a chimeric antigen receptor (CAR) consisting of an antiCD19 specific scFv, a CD8α stalk, transmembrane domain and anchor, a 41BB endodomain and a CD3 zeta endodomain, under the control of a human PGK1 promoter and a modified Woodchuck post transcriptional response element. The vector genome also contains a 5' terminal CMV enhancer/promoter, a Ψ packaging signal, a Rev response element (RRE) and a central polypurine tract (cPPT).
Структура
Иностранные названия
- Obecabtagenum autoleucelum (латинское)
- Obecabtagene autoleucel (английское)
- Obecabtagen autoleucel (немецкое)
- Obécabtagène autoleucel (французское)
- Obecabtagén autoleucel (испанское)
Подробнее по теме
Узнайте дополнительные сведения о действующем веществе Обекабтаген аутолейцел:
